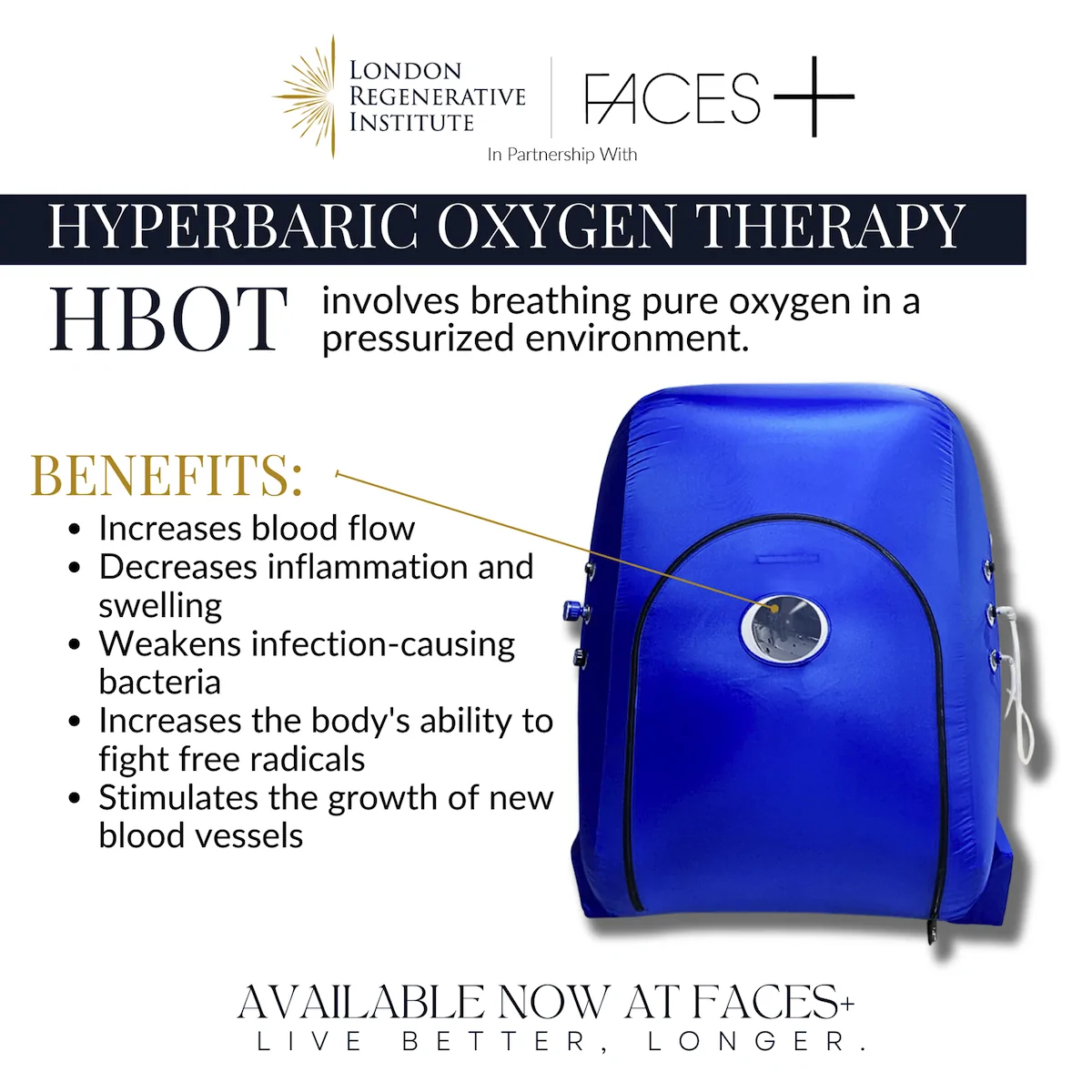
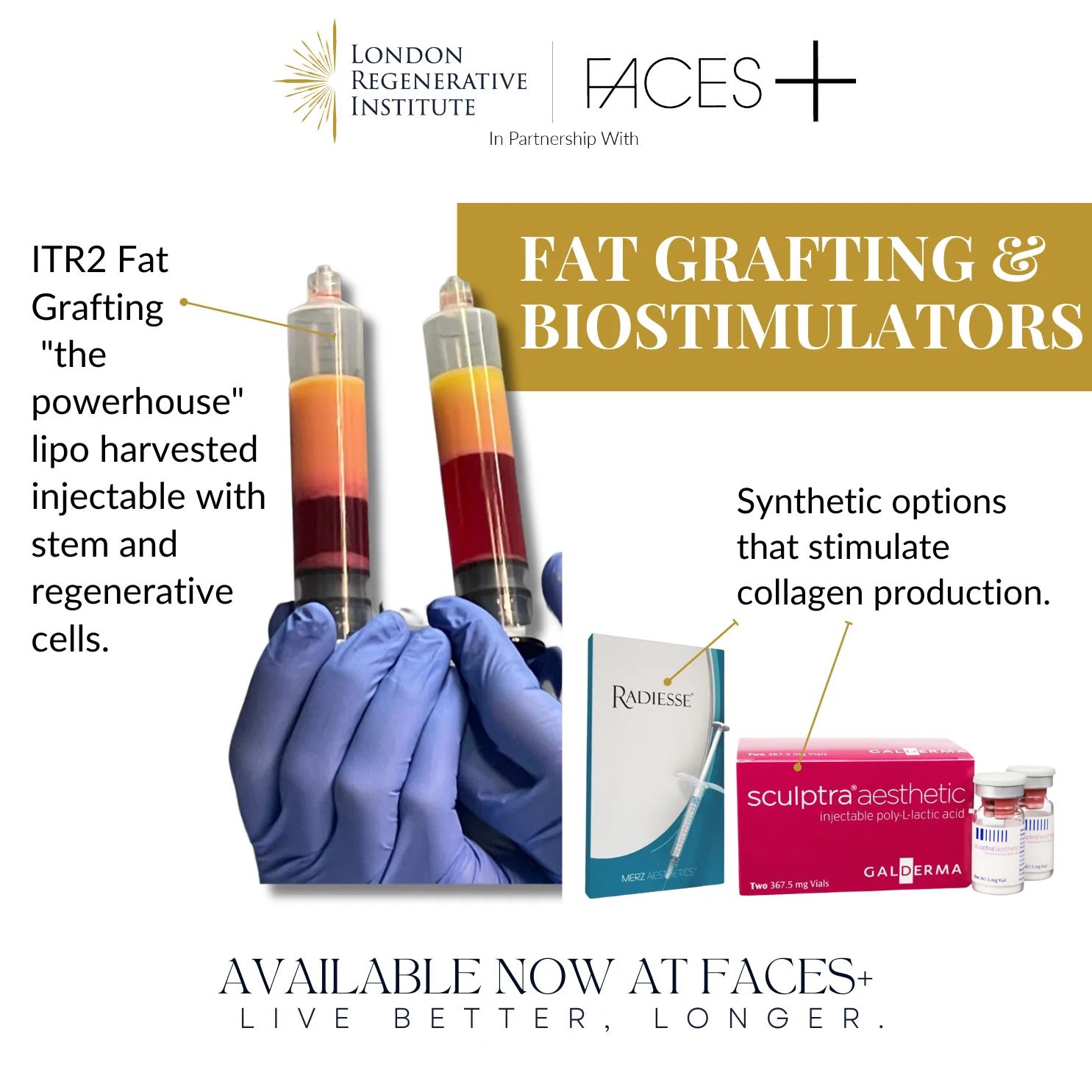
Additional Procedures
The use of adipocyte-derived regenerative and stem cells (ADRC’s) is not presently approved by the FDA. In addition, the major Plastic Surgery Societies have recently issued cautionary statements regarding the use of stem cells in cosmetic surgery. First of all, most surgeons claiming the use of stem cells are simply transferring fat and two, we really do not know the full implications of stem cells and there is particular concern about their use in the female breast.
Although virtually all published data has indicated the safety of ADRC’s produced by standardized lab equipment, the data is still small (approximately 6000 patients have been operated on using this exact procedure and process). Nevertheless, there is compelling case report information in some areas of medicine including plastic surgery that demonstrate the effects of ADRC’s in radiation injuries, inflammatory bowel disease, reconstructive and aesthetic breast surgery and facial reconstruction as well as burns that make it necessary to continue to evaluate the potentially enormous benefits of using our bodies own repair and protective mechanisms to assist in reconstruction and repair.
Dr. Cohen is presently studying the outcomes of these techniques under an IRB study protocol, but is no longer enrolling patients into the study. Dr. Cohen is also one of the founders and is executive director of the Cell Society, an international, non-profit organization dedicated to the promotion and advancement of regenerative cell therapies. The Cell Society’s mission is to expand the global use of cell therapies in a number of medical specialties and to support the clinicians who use them. Dr. Cohen is also a part time employee of Cytori Therapeutics, a local San Diego company evaluating the use of ADRC’s in cardiac disease, burns and orthopedics as well as operating commercially under a CE mark in Europe.